Body composition between obstructive and non-obstructive bladder cancer: A retrospective study
DOI:
https://doi.org/10.46475/asean-jr.v25i2.896Keywords:
Bladder cancer, Body composition, Computed tomography, Skeletal muscle, Subcutaneous fat, Visceral fatAbstract
Background: Body composition measurement during cancer follow-up would increase its role in improving nutritional status. Using a CT scan for nutritional evaluation with scheduled cancer screening or follow-up would add other useful information to help the physician gain a better understanding of the patient’s nutritional status, especially in adipose tissue.
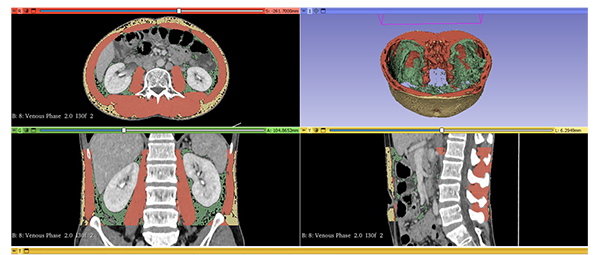
Objective: To evaluate the correlation of measured adipose tissue and skeletal muscle between obstructive and non-obstructive uropathy in bladder cancer on a CT scan.
Background: Body composition measurement during cancer follow-up would increase its role in improving the nutritional status. Using a CT scan for nutritional evaluation with scheduled cancer screening or follow-up would add other useful information to help the physician gain a better understanding of the patient’s nutritional status, especially in adipose tissue.
Objective: To compare the measured adipose tissue and the skeletal muscle between obstructive and non-obstructive uropathy in bladder cancer on a CT scan.
Materials and Methods: A total of 69 patients, who underwent a CT scan of the abdomen including the pelvis before surgery and/or chemotherapy between January 2013 and December 2022, were enrolled. Analyses of the volume of visceral adipose tissue (VAT), subcutaneous adipose tissue (SAT), and skeletal muscle tissue (SMT) calculated based on CT images were performed.
Results: There was significantly lower VAT (p = 0.012) in the obstructive group than in the non-obstructive group. SAT, SMT, age, weight, height, BMI, and tumor size were not significantly different between both groups.
Conclusion: In patients with bladder cancer, those with obstructive uropathy showed lower VAT than non-obstructive uropathy.
Downloads
Metrics
References
Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2021;71:209–49. doi: 10.3322/caac.21660. DOI: https://doi.org/10.3322/caac.21660
Antoni S, Ferlay J, Soerjomataram I, Znaor A, Jemal A, Bray F. Bladder cancer incidence and mortality: A global overview and recent trends. Eur Urol 2017;71:96–108. doi: 10.1016/j.eururo.2016.06.010. DOI: https://doi.org/10.1016/j.eururo.2016.06.010
Baracos VE, Martin L, Korc M, Guttridge DC, Fearon KCH. Cancer-associated cachexia. Nat Rev Dis Primers 2018;4:17105. doi: 10.1038/nrdp.2017.105. DOI: https://doi.org/10.1038/nrdp.2017.105
Grimberg DC, Shah A, Molinger J, Whittle J, Gupta RT, Wischmeyer PE, et al. Assessments of frailty in bladder cancer. Urol Oncol 2020;38:698–705. doi: 10.1016/j.urolonc.2020.04.036. DOI: https://doi.org/10.1016/j.urolonc.2020.04.036
Fukushima H, Takemura K, Suzuki H, Koga F. Impact of sarcopenia as a prognostic biomarker of bladder cancer. Int J Mol Sci 2018;19:2999. doi: 10.3390/ijms19102999. DOI: https://doi.org/10.3390/ijms19102999
Tanaka K, Taoda A, Kashiwagi H. The associations between nutritional status, physical function and skeletal muscle mass of geriatric patients with colorectal cancer. Clin Nutr ESPEN 2021;41:318–24. doi: 10.1016/j.clnesp.2020.11.009. DOI: https://doi.org/10.1016/j.clnesp.2020.11.009
Wells JC, Fewtrell MS. Measuring body composition. Arch Dis Child 2005;91:612–7. doi: 10.1136/adc.2005.085522. DOI: https://doi.org/10.1136/adc.2005.085522
Tan CC, Sheng TW, Chang YH, Wang LJ, Chuang CK, Wu CT, et al. Utilizing computed tomography to analyze the morphomic change between patients with localized and metastatic renal cell carcinoma: body composition varies according to cancerstage. J Clin Med 2022;11:4444. doi: 10.3390/jcm11154444. DOI: https://doi.org/10.3390/jcm11154444
Sanchez A, Kissel S, Coletta A, Scott J, Furberg H. Impact of body size and body composition on bladder cancer outcomes: Risk stratification and opportunity for novel interventions. Urol Oncol 2020 ;38:713–8. doi: 10.1016/j.urolonc.2020.03.017. DOI: https://doi.org/10.1016/j.urolonc.2020.03.017
Borga M, West J, Bell JD, Harvey NC, Romu T, Heymsfield SB, et al. Advanced body composition assessment: from body mass index to body composition profiling. J Invest Med 2018;66:1–9. doi: 10.1136/jim-2018-000722. DOI: https://doi.org/10.1136/jim-2018-000722
Potenta SE, D’Agostino R, Sternberg KM, Tatsumi K, Perusse K. CT urography for evaluation of the ureter. Radiographics 2015;35:709–26. doi: 10.1148/rg.2015140209. DOI: https://doi.org/10.1148/rg.2015140209
Zhao L, Tian X, Duan X, Ye Y, Sun M, Huang J. Association of body mass index with bladder cancer risk: a dose-response meta-analysis of prospective cohort studies. Oncotarget 2017;8:33990–4000. doi: 10.18632/oncotarget.16722. DOI: https://doi.org/10.18632/oncotarget.16722
Rigiroli F, Zhang D, Molinger J, Wang Y, Chang A, Wischmeyer PE, et al. Automated versus manual analysis of body composition measures on computed tomography in patients with bladder cancer. Eur J Radiol 2022;154:110413. doi: 10.1016/j.ejrad.2022.110413. DOI: https://doi.org/10.1016/j.ejrad.2022.110413
Franco-Villoria M, Wright CM, McColl JH, Sherriff A, Pearce MS; Gateshead Millennium Study core team. Assessment of adult body composition using bioelectrical impedance: comparison of researcher calculated to machine outputted values. BMJ Open 2016;6:e008922. doi: 10.1136/bmjopen-2015-008922. DOI: https://doi.org/10.1136/bmjopen-2015-008922
Ying T, Borrelli P, Edenbrandt L, Enqvist O, Kaboteh R, Trägårdh E, et al. Automated artificial intelligence-based analysis of skeletal muscle volume predicts overall survival after cystectomy for urinary bladder cancer. Eur Radiol Exp 2021;5:50. doi: 10.1186/s41747-021-00248-8. DOI: https://doi.org/10.1186/s41747-021-00248-8
Miyake M, Morizawa Y, Hori S, Marugami N, Shimada K, Gotoh D, et al. Clinical impact of postoperative loss in psoas major muscle and nutrition index after radical cystectomy for patients with urothelial carcinoma of the bladder. BMC Cancer 2017;17237. doi: 10.1186/s12885-017-3231-7 DOI: https://doi.org/10.1186/s12885-017-3231-7
Miyake M, Owari T, Iwamoto T, Morizawa Y, Hori S, Marugami N, et al. Clinical utility of bioelectrical impedance analysis in patients with locoregional muscle invasive or metastatic urothelial carcinoma: a subanalysis of changes in body composition during neoadjuvant systemic chemotherapy. Support Care Cancer 2018;26:1077–86. doi: 10.1007/s00520-017-3924-0. DOI: https://doi.org/10.1007/s00520-017-3924-0
Phuong A, Marquardt JP, O’Malley R, Holt SK, Laidlaw G, Eagle Z, et al. Changes in skeletal muscle and adipose tissue during cytotoxic chemotherapy for testicular germ cell carcinoma and associations with adverse events. Urol Oncol 2022;40:456.e19-456.e30. doi: 10.1016/j.urolonc.2022.07.013. DOI: https://doi.org/10.1016/j.urolonc.2022.07.013
Stangl-Kremser J, D’Andrea D, Vartolomei M, Abufaraj M, Goldner G, Baltzer P, et al. Prognostic value of nutritional indices and body composition parameters including sarcopenia in patients treated with radiotherapy for urothelial carcinoma of the bladder. Urol Oncol 2019;37:372–9. doi: 10.1016/j.urolonc.2018.11.001. DOI: https://doi.org/10.1016/j.urolonc.2018.11.001
Rishor-Olney CR, Hinson MR. Obstructive uropathy. [Updated 2023 Jul 22]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2024 Jan- [cited 2024 Jul 4]. Available from: http://www.ncbi.nlm.nih.gov/books/NBK558921/
Jensen GL. Malnutrition and nutritional assessment. In: Loscalzo J, Fauci A, Kasper D, Hauser S, Longo D, Jameson J, editors. Harrison's principles of internal medicine [Internet]. 21th ed. McGraw Hill; 2022 [cited 2024 Jul 4]. Available from: https://accessmedicine.mhmedical.com/content.aspx?bookid=3095§ionid=264532265
Ancoli-Israel S, Bliwise DL, Nørgaard JP. The effect of nocturia on sleep. Sleep Med Rev 2011;15:91–7. doi: 10.1016/j.smrv.2010.03.002. DOI: https://doi.org/10.1016/j.smrv.2010.03.002
Wensveen FM, Valentić S, Šestan M, Turk Wensveen TT, Polić B. Interactions between adipose tissue and the immune system in health and malnutrition. Semin Immunol2015;27:322–33. doi: 10.1016/j.smim.2015.10.006. DOI: https://doi.org/10.1016/j.smim.2015.10.006
Lyon TD, Frank I, Takahashi N, Boorjian SA, Moynagh MR, Shah PH, et al. Sarcopenia and response to neoadjuvant chemotherapy for muscle-invasive bladder cancer. Clin Genitourin Cancer 2019;17:216-22.e5. doi: 10.1016/j.clgc.2019.03.007. DOI: https://doi.org/10.1016/j.clgc.2019.03.007
Wang Y, Chang A, Tan WP, Fantony JJ, Gopalakrishna A, Barton GJ, et al. Diet and exercise are not associated with skeletal muscle mass and sarcopenia in patients with bladder cancer. Eur Urol Oncol 2021;4:237–45. doi: 10.1016/j.euo.2019.04.012. DOI: https://doi.org/10.1016/j.euo.2019.04.012
Downloads
Published
How to Cite
Issue
Section
License
Copyright (c) 2024 The ASEAN Journal of Radiology

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.
Disclosure Forms and Copyright Agreements
All authors listed on the manuscript must complete both the electronic copyright agreement. (in the case of acceptance)
















